
- Investment
- 科学论坛
-
Program
- Recruitment
- Center
News Message
To Boost Lithium-Ion Battery Capacity by up to 70 percent
- by wittx 2020-09-04

There was a time when budding inventors were advised to build a better mousetrap. Nowadays, they’d do rather well to build a better lithium-ion battery. These are what power our phones, laptops, portable power tools, an increasing number of cars, even homes. Some places are turning to giant lithium-ion batteries to store energy from solar panels so that it can be used after dark. While lithium-ion cells have gotten incrementally better over the years, they seem set for a big boost in 2019 through the increased use of an element not unfamiliar to the electronics industry: silicon.
The reason lies in some fundamental electrochemistry. Lithium-ion cells work by sending lithium ions from the positive electrode (in a battery, it’s called the cathode) to the negative electrode (the anode) during charging. During discharge, lithium ions move in the opposite direction, from anode to cathode. So charging such a battery amounts to storing lithium in the anode. If your battery could store more lithium, it would store more energy.
In the garden-variety lithium-ion battery used in smartphones, laptops, and most electric cars, the anode is made of graphite, a form of carbon. Lithium is stored in the electrode in the form of LiC6, in which one lithium atom is surrounded by six carbon atoms.
Battery developers have been trying for years to figure out how to use silicon instead of carbon in anodes, because lithium ions combine with silicon to form Li15Si4. The 15-to-4 ratio means a smaller amount of anode material can store a lot more lithium. Silicon anodes could thus provide much larger capacities.
The rub is that silicon expands almost 300 percent in volume when it reacts with lithium during charging. It then shrinks by the same amount during discharge. Repeated charge-discharge cycling causes the anode to begin to disintegrate. That in turn creates more surface area on the anode, which then reacts chemically with the electrolyte, damaging the battery. So batteries with silicon anodes tend not to hold up for long.
Happily enough, silicon’s expansion problem is not insurmountable. Even now, some lithium-ion batteries have anodes that include particles containing silicon combined with silicon dioxide (the stuff of sand) and coated with carbon. Elon Musk revealed in 2016 that the Tesla’s lithium-ion cells are built that way. But to date, the amount of silicon in anodes has been minimal.
Expect that to change in 2019. To begin with, a California startup named Sila Nanotechnologies plans to commercialize a silicon-rich anode material. Company cofounder and Georgia Tech professor Gleb Yushin says that Sila has developed a “drop-in solution” for existing battery manufacturers, which is slated to go into commercial production in 2019.
Sila’s Silicon Savior: These prototype cells, built with a silicon-rich anode material developed by Sila Nanotechnologies, help demonstrate a new approach for boosting the capacity of lithium-ion batteries. Depending on the application, use of this anode material will boost battery capacity initially by about 20 percent and eventually by 40 percent or better. What’s more, explains Yushin, it allows the anode to be reduced in thickness by up to 67 percent, which in turn may permit the battery to be charged as much as nine times as fast. And it brings safety benefits as well, he claims, because it suppresses the formation of threadlike metallic dendrites, which can cause cells to short out internally and burst into flame.
Yushin says his company’s new anode material is composed of particles that are similar in size to the graphite ones being used in anodes now. But they contain silicon inside a porous scaffolding, which provides room for the silicon to expand and contract without coming into contact with the electrolyte. This allows batteries made with this silicon-rich anode material to perform well for 400 to 1,000 full charge-discharge cycles, which is more than enough for most applications. “Even for electric cars, you often don’t need more than 1,000 cycles,” says Yushin.
That helps explain the interest of BMW, which is working with Sila to explore whether lithium-ion batteries built with the new anode material can be used in its electric cars. Nevertheless, Yushin says “the initial products will be wearables,” for which the cost of the battery is not such a critical factor and the amount of anode material required is much more modest, meaning that his company can more easily meet demand. Yushin expects lithium-ion batteries with Sila anodes will be in millions of devices in 2019.
Sila probably won’t be the only company to unveil a silicon-battery technology this year. Another California company, Enovix, is expected to introduce an anode that is made entirely of silicon and silicon oxides.
Ashok Lahiri, cofounder and chief technology officer for Enovix, along with two colleagues, described the company’s battery technology in detail in these pages in 2017. At the time, Enovix planned to borrow fabrication techniques from the semiconductor industry to construct batteries from thin wafers of solar-grade silicon. But the company reconsidered that strategy after grappling with how to apply it to larger lithium-ion batteries for vehicles. “We realized that the solar-grade substrates could not scale,” says Lahiri.
So Enovix revamped its approach and is now using a metal foil instead of a silicon wafer as the substrate for its battery. The overall geometry of the battery, however, remains the same. It’s just built differently—by stacking components, says Lahiri, who explains that keeping the anode stack under high pressure inhibits the expansion during charge and allows the anode to be made entirely from silicon and silicon oxides.
“We think our battery will be from 30 to 70 percent better
Share Http URL: http://www.wittx.cn/get_news_message.do?new_id=195

Best Last Month
Information industry by wittx

Information industry by wittx
Information industry by wittx
Information industry by wittx
Information industry by wittx
Information industry by wittx
Information industry by wittx

Information industry by show
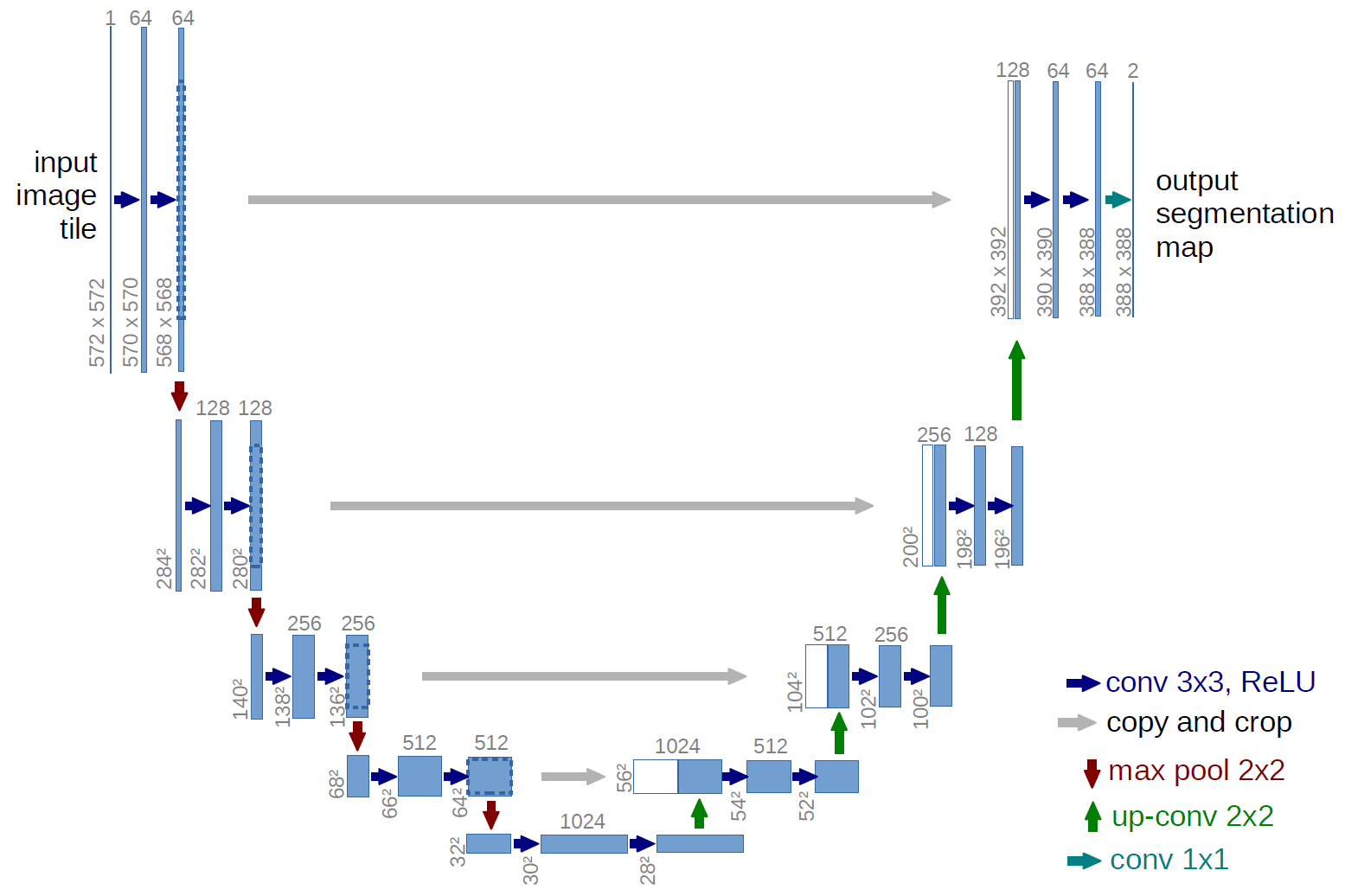
Information industry by wittxHigh-Resolution Model for Segmenting and Predicting Brain Tumor Based on Deep UNet with Multi Attent
Traffic by wittxThree Small Stickers in Intersection Can Cause Tesla Autopil
